

The dilution factor after four dilutions is Repeat the process until you have four tubes. Take a 1mM stock solution of adrenaline, dilute it 1:10 (0.1ml + 0.9ml) to give a 0.1mM solution dilute this 1:10 to give a 0.01mM solution dilute this 1:10 to give a 0.001mM solution dilute this 1:10 to give a 0.0001mM solution and so on This approach allows you to prepare very dilute solutions from a concentrated. Then transfer 0.2 mL from Tube 2 to 3.8 mL of diluent in Tube 3 and mix. You would transfer 0.2 mL from Tube 1 to 3.8 mL of diluent in Tube 2 and mix. So you multiply each successive dilution by the dilution factor.
How to do dilution series math serial#
Remember that serial dilutions are always made by taking a set quantity of the initial dilution and adding it successively to tubes with the same volume. If you did the above dilution four times, what would be the final dilution factor? What is the dilution factor if you add 0.2 mL of a stock solution to 3.8 mL of diluent? This Webinar is intended to give a brief introduction into the mathematics of making solutions commonly. If there is 1g in 50mL, there is 2g in 100mL.
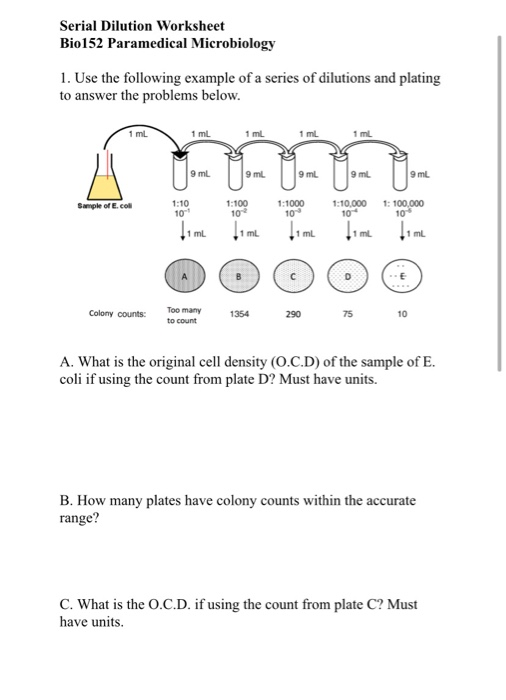
Find the concentration of the diluted product as a percentage strength, a ratio strength and an amount strength expressed as mg/mL. #DF = V_i/V_f# = #(1"mL")/(10"mL") = 1/10#. Webinar on Laboratory Math II: Solutions and Dilutions. 100mL of a 1 in 50 w/v solution is diluted to 1000mL. The dilution factor or the dilution is the initial volume divided by the final volume.įor example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, In serial dilutions, you multiply the dilution factors for each step. For example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, DFViVf 1mL10mL110. By using a stock, a medicine can be prepared by simple dilution. The dilution factor or the dilution is the initial volume divided by the final volume. Stock solutions are concentrated solutions. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. In serial dilutions, you multiply the dilution factors for each step.


 0 kommentar(er)
0 kommentar(er)
